Categories
- Shop All (4980)
-
-
- Hi-Vis Chainsaw Safety (14)
- Hi-Vis Coveralls and Overalls (10)
- Hi-Vis Hoodies and Shirts (27)
- Hi-Vis Jackets (30)
- Hi-Vis Pants (14)
- Hi-Vis Rain Wear (30)
- Hi-Vis Vests (36)
- Winter Hi-Vis Bombers and Parkas (18)
- Winter Hi-Vis Coveralls and Overalls (18)
- Winter Hi-Vis Hoodies (3)
- Winter Hi-Vis Pants (3)
- Winter Hi-Vis Vests (5)
-
-
-
-
- Brooms (2)
- Dust Mops (0)
- Dust Pans and Brushes (4)
- Flow Thru Tools (2)
- Microfiber Mops (0)
- Pool and Tank Tools (2)
- Scrubbers and Scrapers (1)
- Soap and Sanitizer Dispensers (0)
- Sprayers (1)
- Squeegees (1)
- Toilet Brushes and Plungers (3)
- Trash Cans & Bags (8)
- Wet Floor Signs (0)
- Wet Mops and Buckets (4)
-
- Bars and Prying Tools (21)
- Bolt Cutters and Shears (17)
- Chisels and Punches (9)
- Combination Hand Tool Sets (12)
- Extractors (13)
- Files (6)
- Gear Pullers (14)
- Hammers and Mallets (14)
- Hand Saws (17)
- Hex Keys (12)
- Layout and Distance Lasers (1)
- Marking Tools (0)
- Measuring Tools (10)
- Pliers (52)
- Precision Measuring and Testing Tools (24)
- Propane Torches (5)
- Screwdrivers and Nutdrivers (21)
- Sockets (66)
- Tap and Die Sets (11)
- Tool Boxes (26)
- Utility Knives (14)
- VDE Tools (7)
- Wire Cutters and Strippers (14)
- Wrenches (22)
-
- Beveling and Deburring (17)
- Curb and Valve Keys (20)
- Drilling and Tapping (12)
- Extended Impact Sockets (3)
- Flaring and Rerounding (7)
- General Pipe Working Tools (19)
- Guillotine Pipe Cutters (2)
- Hydrostatic Test Pumps (14)
- Internal Pipe Cutters (6)
- Manhole Testing (4)
- PE Peelers (8)
- Pipe Reamers (4)
- Pipe Threading (17)
- Pipe Wrenches (24)
- Plastic Pipe Joint Kits (4)
- Plastic Pipe Saws (5)
- Power Drive (13)
- Quick Release Cutters (15)
- Ratchet Shears (9)
- Ratcheting Wrenches (12)
- Rotary Cutters (3)
- Shut Off Tools (9)
- Soldering Torches (3)
- Vises (7)
-
- Angle Grinders (6)
- Batteries and Chargers (18)
- Bench Grinders (7)
- Circular Saws (3)
- Combo Tool Kits (10)
- Cordless Fans (6)
- Cordless Lighting (15)
- Cut Off Saws (4)
- Drills and Drivers (8)
- Grease Guns (3)
- Impact Drivers (5)
- Jobsite Radios and Speakers (8)
- Lifestyle (6)
- Mitre Saws (2)
- Reciprocating Saws (4)
-
- Angle Grinder Wheels and Brushes (22)
- Bench Grinder Wheels (7)
- Circular Saw Blades (11)
- Drill and Driver Bits (26)
- High Speed Gas Saw Blades (3)
- Holesaws (11)
- Impact Sockets (25)
- Jig Saw Blades (2)
- Oscillating Multi Tool Blades (1)
- Portable Chop Saw Blades (4)
- Power Tool Storage (5)
- Reciprocating Saw Blades (8)
-
-
-
-
- Air Fresheners (0)
- All Purpose Cleaners (19)
- Bowl and Washroom (8)
- Coffee and Breakroom (19)
- Degreasers (3)
- Dishwashing (4)
- Disinfectants and Sanitizers (1)
- Drain Openers (7)
- Hand Cleaners (8)
- Laundry Cleaners (9)
- Paper Products and Wiper Rags (18)
- Scale Removers (4)
- Urinal Pucks and Liquids (5)
- Wet Wipes (2)
-
-
- Ball Valves (13)
- Black and Galvanized Steel Fittings (16)
- Bronze Pipe Fittings and Nipples (13)
- Butterfly Valves (4)
- Check Valves (18)
- Flexible Connectors (4)
- Gate and Globe Valves (5)
- Knife Gate Valves (9)
- Pipe Fitting Accessories (4)
- Schedule 80 PVC Fittings (27)
- Stainless Steel Fittings and Valves (24)
- Victaulic Grooved Fittings (22)
Lovibond - Buffer Solutions
4 products
Showing 1 - 4 of 4 products
What Are Lovibond Buffer Solutions?
Lovibond provides highly reliable tools for ensuring accurate water quality analysis. Known for its innovation and scientific rigour, the company offers a wide range of products, including pH buffer solutions that support precise calibration and maintenance of pH meters. These solutions are designed for both laboratory and field use, with NIST traceability, colour coding, and practical packaging that simplify identification and handling. By maintaining electrode accuracy and consistency, Lovibond products help users achieve dependable water quality monitoring across various applications.
Why Choose Lovibond - Buffer Solutions?
Lovibond Buffer Solutions offer dependable pH calibration with clearly defined values and excellent shelf stability. Designed for consistent performance, they meet international standards and are suitable for a wide range of testing environments, from routine lab use to demanding field applications. Explore these buffer solutions at our Canadian warehouse, or you can check them out below:
-
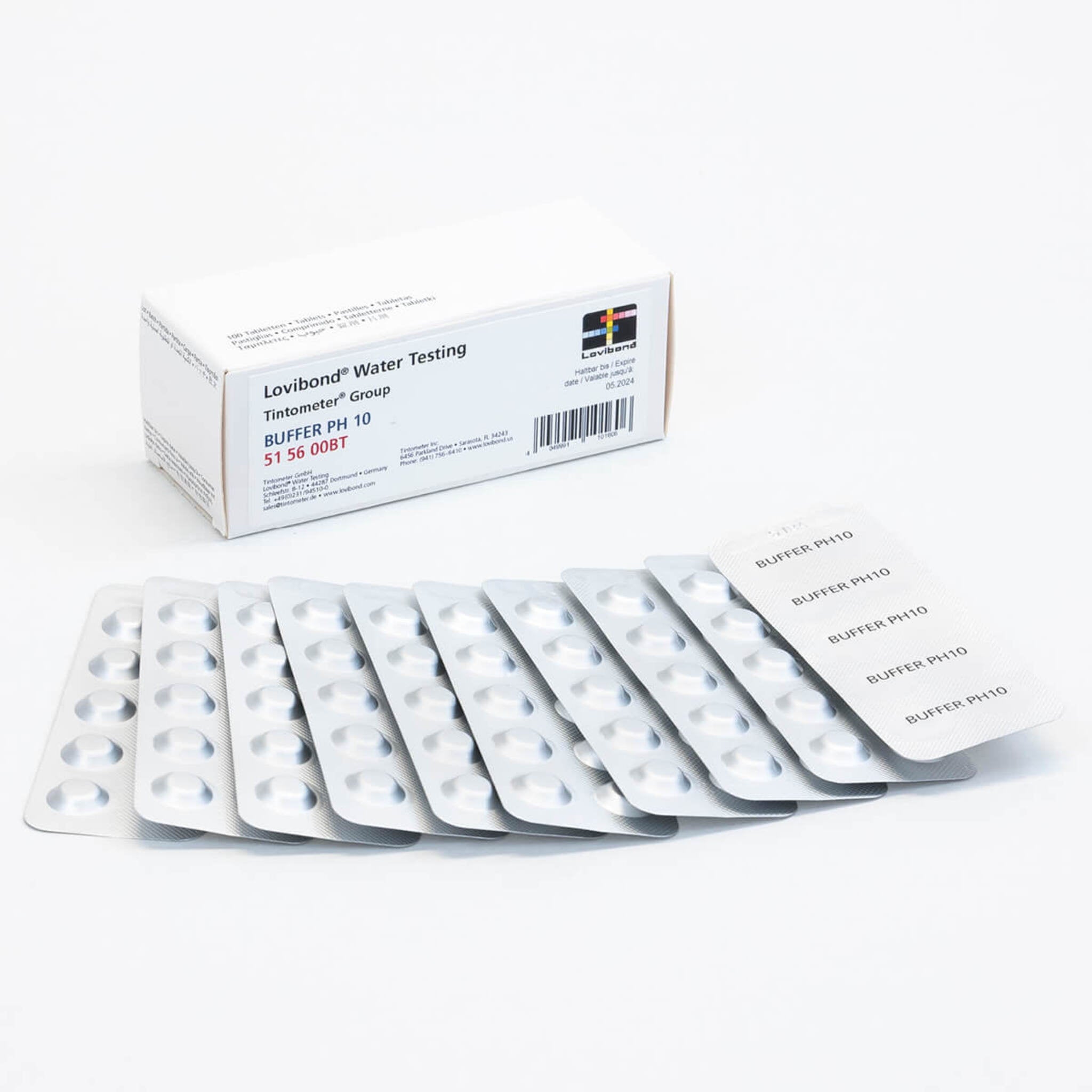
Lovibond pH Buffer Tablets
-
Lovibond pH Buffer Tablets offer a convenient and eco-friendly way to prepare fresh calibration solutions for accurate pH measurements. Simply dissolve a tablet in 20 ml of de-ionized water for a quick, colour-indicated pH solution. Packaged in durable aluminum blister packs, the tablets boast a long shelf life and are ideal for reducing packaging waste. Perfect for lab or field use, they ensure consistent and reliable pH calibration anytime.
-
Lovibond pH 10.01 Buffer Solution
-
Lovibond pH 10.01 Buffer Solution offers precise calibration for all pH meters, supporting both automatic and manual adjustments. Certified and traceable to NIST standards, it ensures accurate, reliable results in lab and field applications. The blue colour allows easy identification, while each 500 ml bottle has an 18-month shelf life when sealed and stored between 5–25°C.
-
Lovibond pH 7.00 Buffer Solution
-
Lovibond pH 7.00 BufferSolution is a certified, NIST-traceable calibration standard designed for precise and reliable pH meter calibration. Compatible with all pH meters—whether using automatic buffer recognition or manual adjustment—it ensures accurate results across laboratory and field applications. The distinct yellow colour aids in easy identification, and each 500 ml bottle offers a 24-month shelf life when stored between 5–30°C.
FAQs
1. Why do buffer solutions resist changes in pH even after adding strong acids or bases?
Buffer solutions resist pH changes because they contain a weak acid and its conjugate base (or a weak base and its conjugate acid) that work together to neutralize added H⁺ or OH⁻ ions. When a strong acid is added, the base component of the buffer reacts with the excess hydrogen ions. When a strong base is introduced, the acid component donates hydrogen ions to neutralize hydroxide ions. This equilibrium allows the solution to maintain a relatively stable pH.
2. Why does the buffering capacity weaken when a buffer is too diluted or overly concentrated?
The buffering capacity weakens when a buffer is too diluted because there are fewer acid–base pairs available to neutralize added acids or bases. On the other hand, an overly concentrated buffer can increase the ionic strength of the solution, which may interfere with chemical or biological processes. An appropriate concentration ensures effective pH stabilization without unwanted side effects.
3. Is it possible to make a buffer solution with a strong acid or base?
No, buffer solutions cannot be made using only strong acids or bases because they dissociate completely in water and do not establish the equilibrium needed to resist pH changes. Buffers require a weak acid and its conjugate base or a weak base and its conjugate acid. For example, acetic acid and sodium acetate form a buffer, whereas hydrochloric acid and sodium chloride do not.
4. What happens if you exceed a buffer’s capacity during a chemical reaction?
When a buffer’s capacity is exceeded, it can no longer maintain a stable pH because all available buffering components have been consumed. Any additional acid or base added will cause a sharp change in pH. This condition, known as buffer exhaustion, can lead to inaccurate results or disrupt pH-sensitive chemical and biological reactions.
5. Can a buffer solution be reused in multiple experiments, or does it degrade over time?
Buffer solutions can sometimes be reused, but their effectiveness may decrease over time due to contamination, evaporation, or gradual pH drift. Repeated exposure to air, microbial growth, or improper storage can alter the buffer’s composition. For best results, fresh buffers should be prepared when accuracy is critical, or existing buffers should be stored in clean, sealed containers under appropriate conditions.











